
Tosoh Bioscience, Inc., based in South San Francisco, CA is a U.S. subsidiary of Tosoh Corporation’s Bioscience Division, headquartered in Tokyo, Japan. Established in the United States in 1989, Tosoh Bioscience has become known throughout the Americas for providing sophisticated diagnostic systems to hospitals and reference laboratories.
Diagnostic Leadership
Tosoh builds upon its proven reputation and maintains leadership in Clinical Diagnostics. With state-of-the-art immunoassay and HPLC systems, Tosoh offers superior instrumentation to continually meet the operational and economic needs of laboratories today and in the future.
Immunoassay Pioneers
Tosoh, a pioneer in the field of automated immunoassay systems, continually evolves its product line to offer the most advanced systems in the world. Tosoh AIA Automated Immunoassay Analyzers provide testing for a broad menu of quality assays in a unique patented dry reagent format.

AIA®-360 Automated Immunoassay Analyzer
Compact Immunoassay Analyzer for Flexible Testing

RELIABLE
- Calibration stability: 90 days
- Biotin-free tests = no biotin interference
- Error-free: automatically checks if reagent cup corresponds
to test required - 24/7 testing reliability
- Traceability of all samples and data
SIMPLE
- Start up in less than 10 minutes
- Friendly operator interface
- Limited maintenance
- No need for test programming
- Easily transportable
- Connected to LIS or similar
COMPACT
- Compact for small places
- No computer
- Includes printer and prints patient results in real time for easy review and traceability, with or without LIS connection
- Can run just about anywhere
- Common reagents easily accessible from the tray attached to the instrument
- Sensors signal when reagents running low or when waste needs to be emptied
EFFICIENT
- Set up in 5 minutes
- Always ready to run
- 1 AIA-PACK® = 1 test
- Real throughput of 36 tests/hour
- Continuous loading of additional samples and tests without interrupting processing
- Handle primary samples: load bar-coded primary tubes and test cups directly into the system
- Sample handling time in less
than 1 minute - Delivers results in real time
- Quality design
Unique Tosoh Reagent Benefits
- Single, unitized cups use a dry reagent format that requires no pre-mixing, no pre-measuring, and minimizes preparation time and waste
- Dry reagent format ensures calibration stability of up to 90 days for most assays
- Rapid turn-around time (20 minutes) of ST AIA-PACK assays provides actionable information for medical decisions.
- The AIA-PACK technology utilizes a patented concept by Tosoh based on individual test cups, each containing lyophilized and ready-to-use reagents.
- No pre-mixing, no pre-measuring, and no on-board refrigeration required.
- One test, one cup ensures minimal reagent waste leading to both increased efficiency and cost savings.
- The entire immunoassay reaction takes place in the test cup, from the pipetting of the sample, the antigen-antibody reaction, up to the signal detection ensuring consistency and stability.
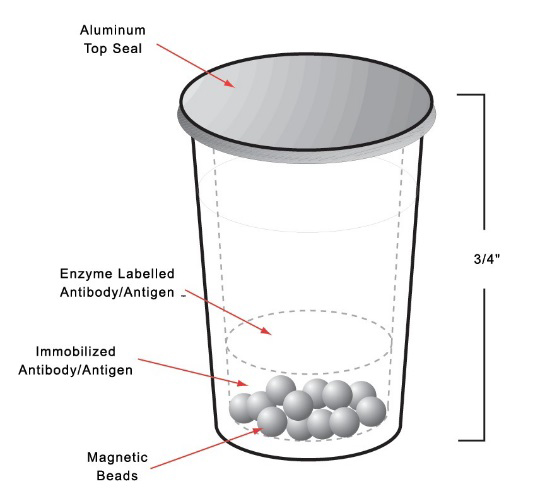
Consistency
The Unit Dose Test Cup technology ensures no transfer of reagents, no pre-mixing and no pre-measuring, minimizing the introduction of analytical errors while maximizing on performance specifications. Together, this yields reliable diagnostic results allowing for accurate clinical decisions and customized patient care.
AIA-PACK assays have excellent CVs across the entire test menu.
Precision of the AIA Pack Immunoassay Menu

*Some products may not be authorized for sale in Canada. Please contact Somagen for specific enquiries.

