Total Lyme Testing Solutions
Gold Standard Diagnostics New B. burgdorferi lgG/IgM VlsE-OspC Assay
Gold Standard Diagnostics’ newest Lyme assay includes the recombinant antigens VIsE and OspC to achieve excellent sensitivity and specificity; the sensitivity is comparable to a standard first tier screening assay and the specificity is comparable to an immunoblot.
The B. burgdorferi lgG/IgM VIsE-OspC assay uses the GSD Standard Lyme EIA protocol with a 15/15/15 incubation.
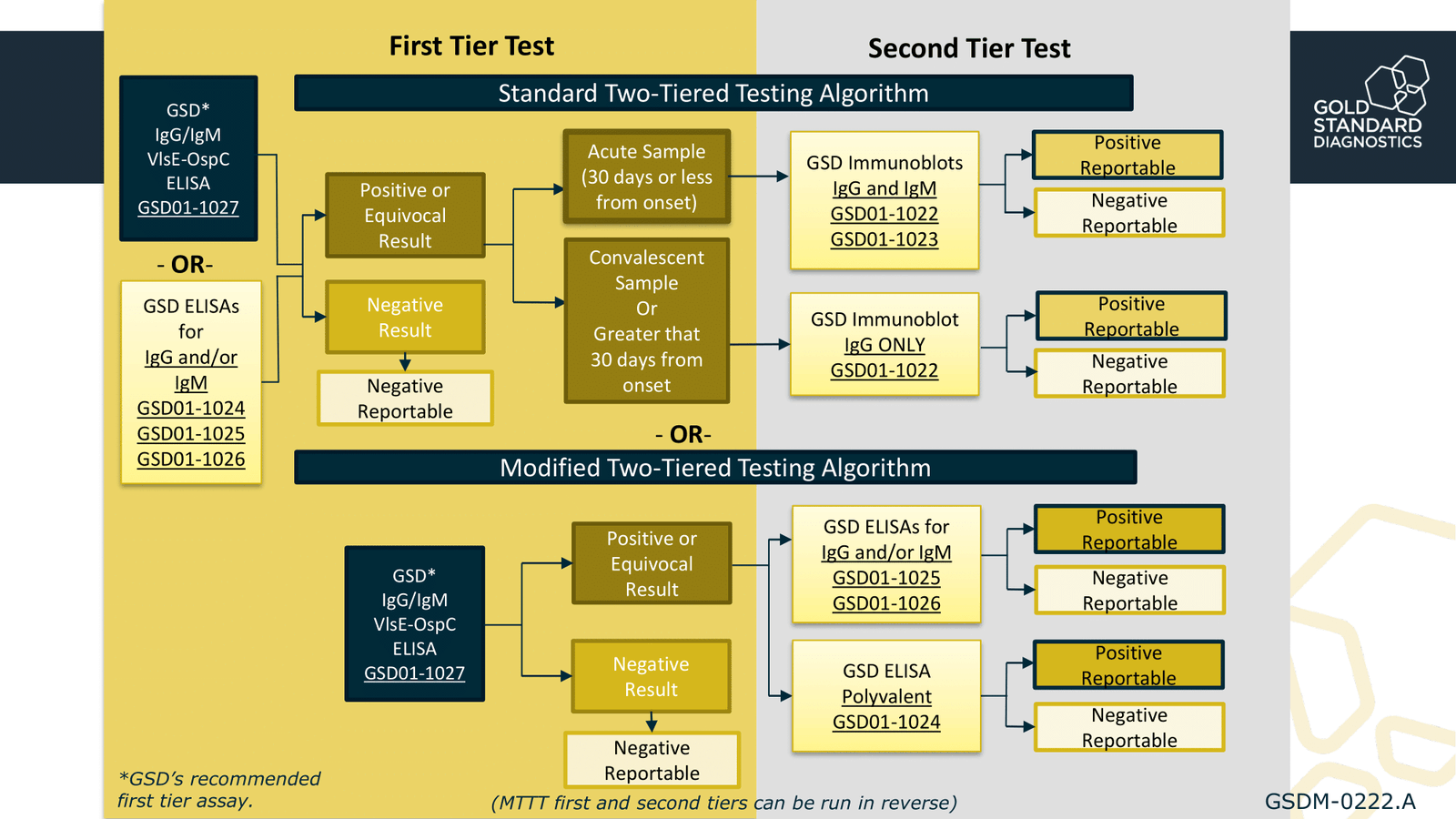
The Modified Two-Tiered Testing algorithm (MTTT) for Lyme Disease diagnosis is based on two EIA tests. This is an alternate option to the Standard Two-Tiered Testing algorithm (STTT) in which a positive screen result is confirmed with an immunoblot.
Using the B. burgdorferi lgG/IgM VlsE-OspC test as the first tier in combination with a second GSD Lyme EIA as the second tier in the MTTT algorithm, we maintain > 98% specificity while demonstrating a nearly 20% increase (1) in Early Lyme Disease detection compared to the STTT.
1. Gold Standard Diagnostics IFU (Instructions For Use) GSD01-1 027 Method Comparison: CDC Reference Panel for Clinical Sample results

All Things Lyme
Gold Standard Diagnostics is the only company offering kits to fulfill Lyme testing requirements for both the Standard and Modified two-tiered testing algorithms. Their four EIA kits and two lmmunoblot kits allow flexibility and testing can be fully automated on open processing platforms.

GSD Whole-Cell Sonicate ELISA Kits
SUPERIOR SENSITIVITY
Includes antigens from B. burgdorferi strains B31 and 2591.
OPTIMAL SPECIFICITY
Contains VIsE immunogenic lipoprotein.
CONVENIENT
Kit includes ready to use controls for ease of use.
AUTOMATABLE
Simple procedure is easy to automate.

GSD Immunoblot Confirmation
NATIVE ANTIGEN STRUCTURE
The 3D (tertiary) antigen structure is maintained, unlike denatured Western blot proteins which only retain primary structure.
IgG AND IgM IMMUNOBLOTS
Separate IgG and IgM immunoblot assays are available containing the CDC recommended specific antigens required for Immunoblot interpretation.
ROBUST STRIPS
Strips are stabilized with a plastic backing and fixed in a booklet, making them easy to handle.
EASILY AUTOMATABLE
Strips are durable, easy to identify, automatable, with an incubation time of 30/30/10.
FAST AND SIMPLE PROCEDURE
Protocol includes 1:100 serum dilution.
INTUITIVE RESULTS
Easy to read, precisely defined results, with internal test controls (serum and conjugate bands) and kit positive, negative, and cut off controls.

